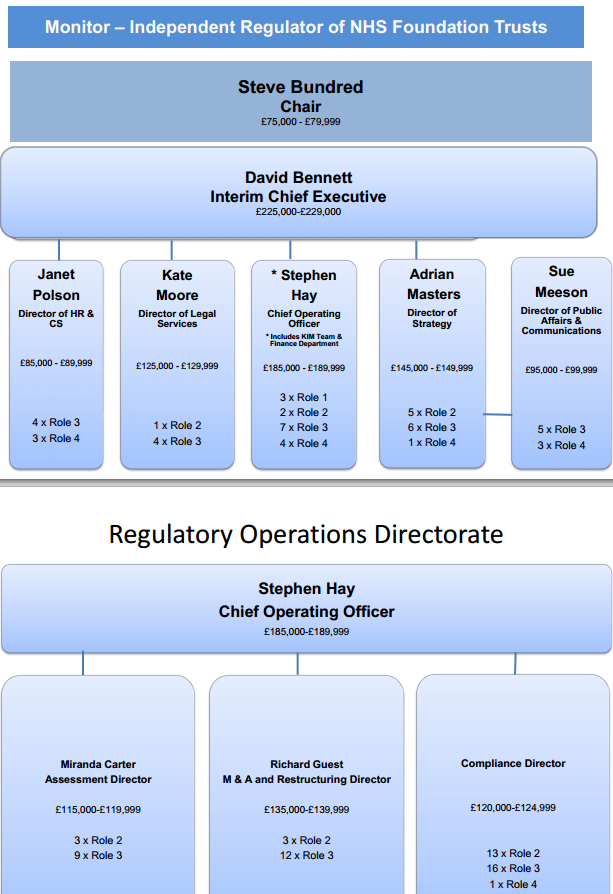
The health regulator Monitor has published data on its structure, including posts, pay scales, and an organogram (PDF) (shown above). The data is particularly useful if you are trying to trace responsibility within the organisation.
If you find the data useful, or need any further help, let us know!